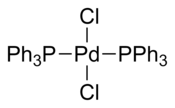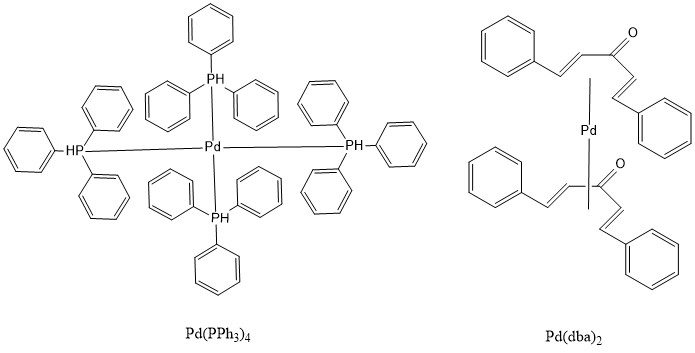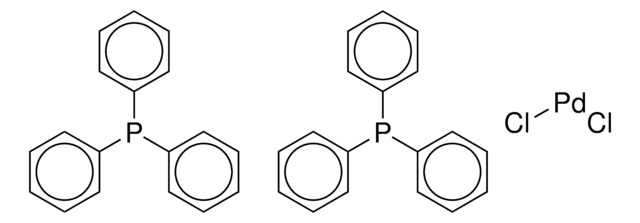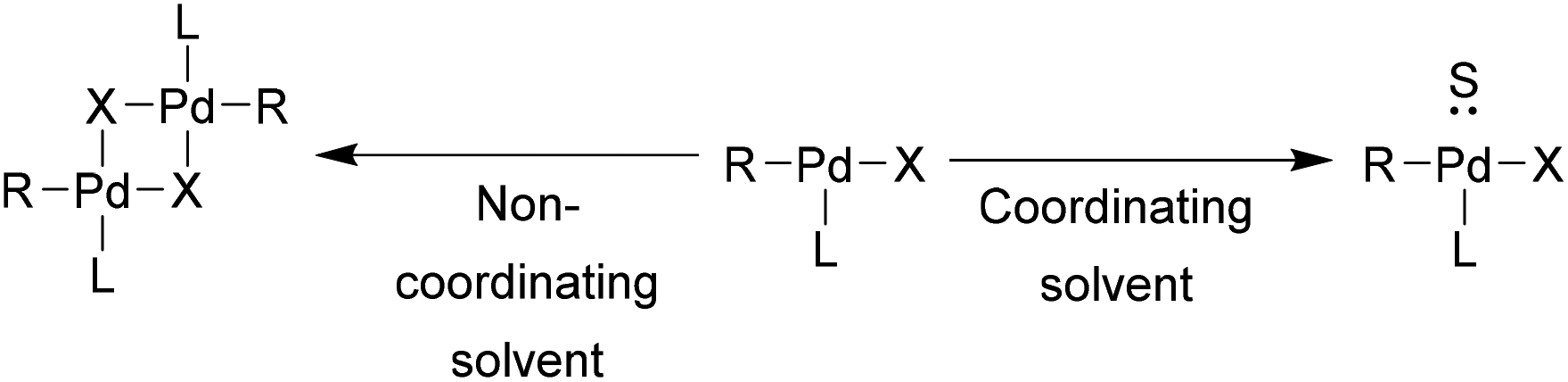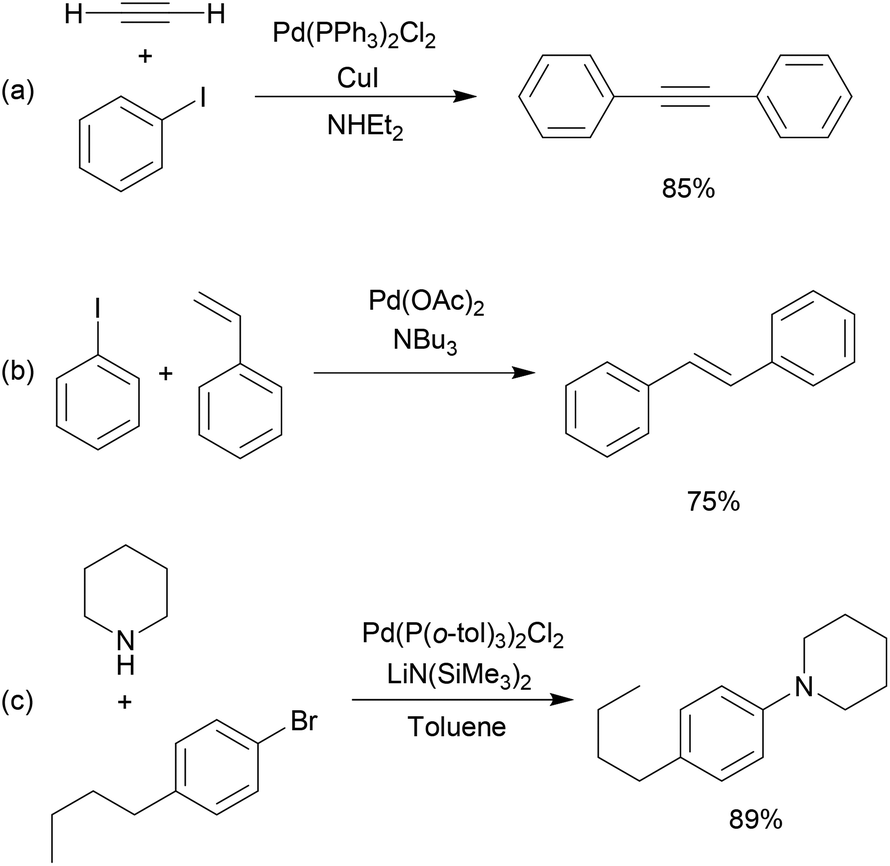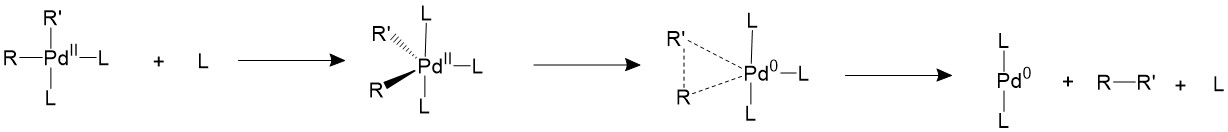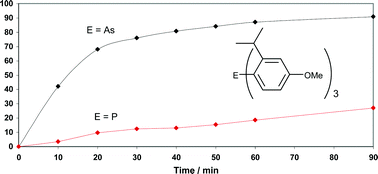
Bulky triarylarsines are effective ligands for palladium catalysed Heck olefination - Dalton Transactions (RSC Publishing)

Synthesis, spectral, X-ray crystallography, electrochemistry, DNA/protein binding and radical scavenging activity of new palladium(II) complexes containing triphenylarsine - ScienceDirect

Synthesis, spectral, X-ray crystallography, electrochemistry, DNA/protein binding and radical scavenging activity of new palladium(II) complexes containing triphenylarsine - ScienceDirect