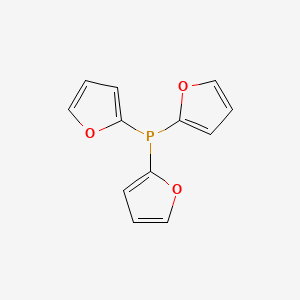
Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect
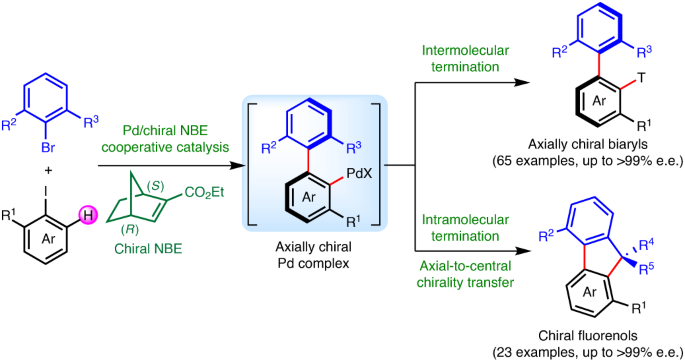
Construction of axial chirality via palladium/chiral norbornene cooperative catalysis | Nature Catalysis
The preparation, resolution and application of novel 2-furyl phosphine ligands in asymmetric synthesis
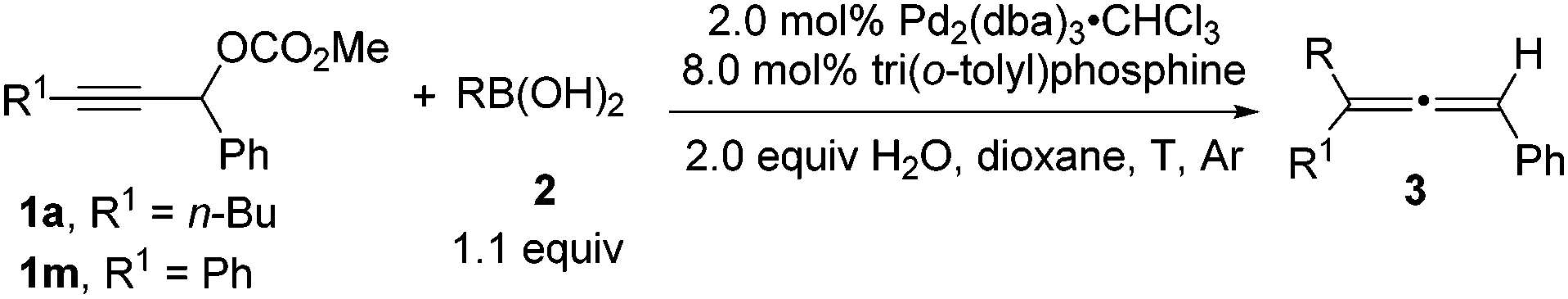
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)
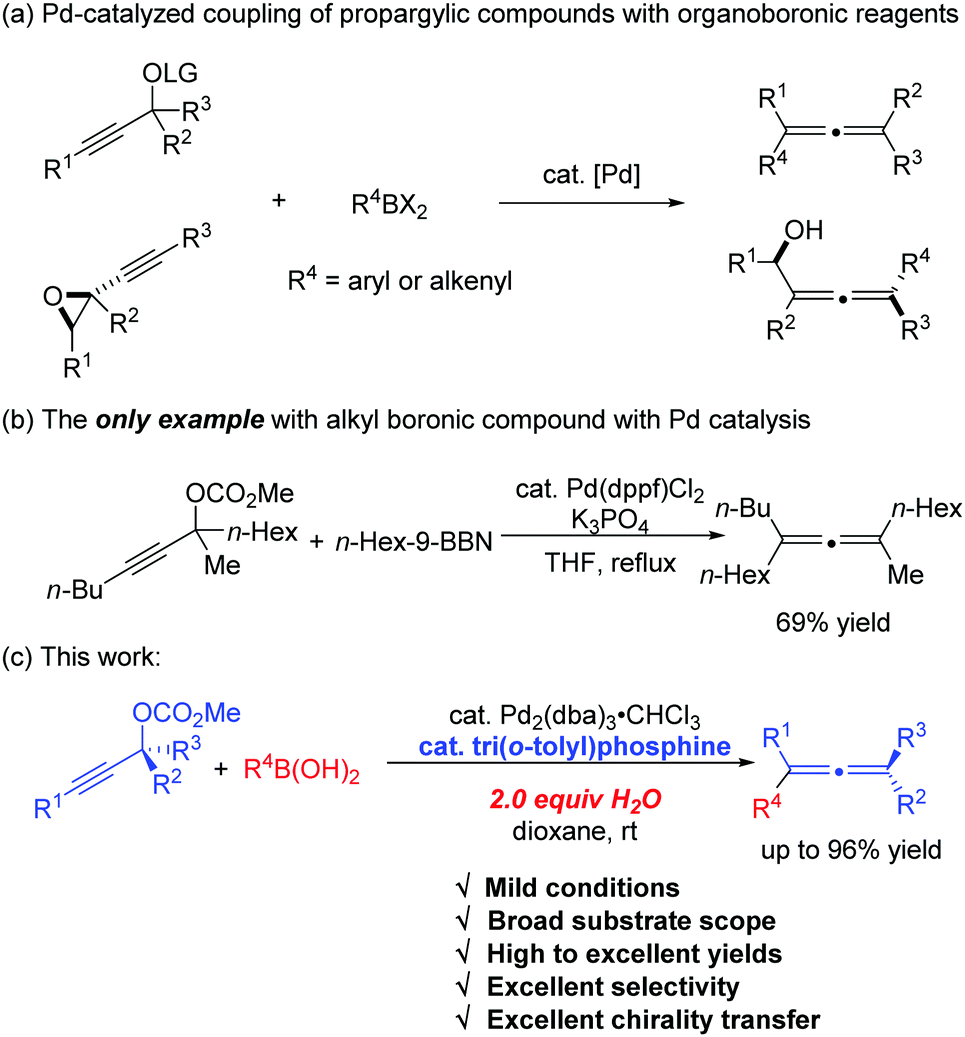
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)

Cationic palladium(II)–acetylacetonate complexes containing phosphine and aminophosphine ligands and their catalytic activities in telomerization of 1,3-butadiene with methanol - ScienceDirect
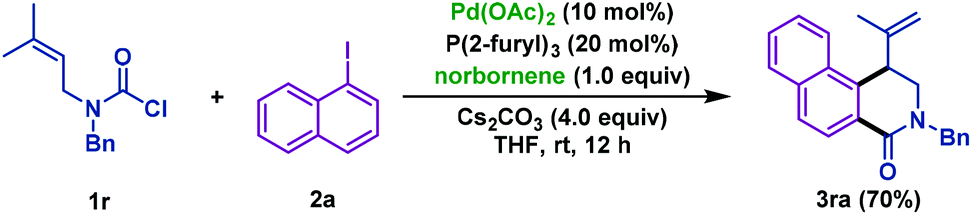
Pd/NBE-catalyzed sequential carbamoylation/olefination of aryl iodides - Organic Chemistry Frontiers (RSC Publishing)
![5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/01/5518-52-5.png)
5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation

China Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) CAS No.: 14221-01-3 Manufacturers - Free Sample - Alfa Chemical

![Phosphine Ligands [Cross-coupling Reaction using Transition Metal Catalysts] | TCI AMERICA Phosphine Ligands [Cross-coupling Reaction using Transition Metal Catalysts] | TCI AMERICA](https://www.tcichemicals.com/medias/T1643.jpg?context=bWFzdGVyfHJvb3R8Mzk2MTR8aW1hZ2UvanBlZ3xoZGMvaDA5Lzg5MzI0ODgyNDkzNzQvVDE2NDMuanBnfDFiZTAzOWNlZGFiNjZjZDMwYzZjZGIzNzY4MWVhMGVkZGEzNTRjMzg0YmVjNDYwNTNlYzk5MzEwOGIyN2EwMDM)